3D Images of Protein Complex May Improve Understanding of Causes of CMT, Other Disorders
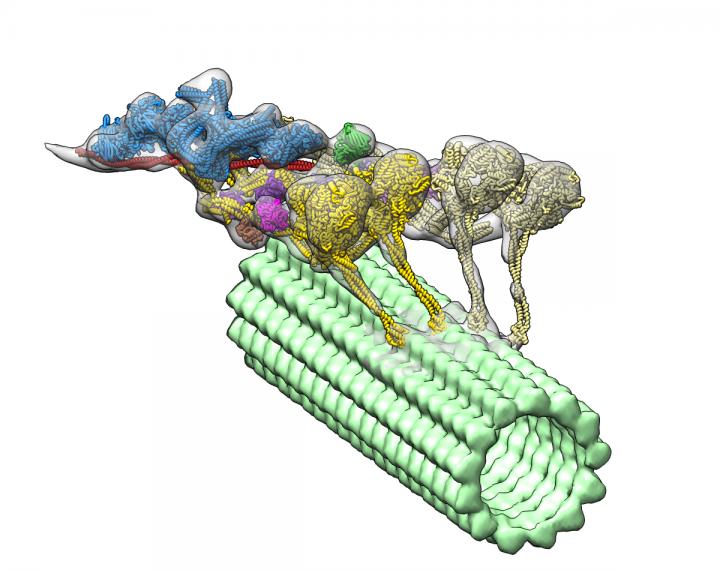
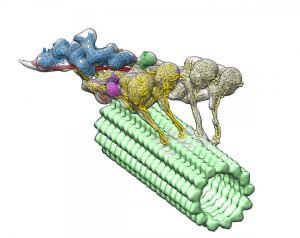
The dynein-dynactin complex. Dynactin is shown in blue; the four motor domains are yellow; and the microtububle is green. Credit: Danielle Grotjohn, Lander Lab
Researchers have revealed 3D images of a critical protein complex that provide an essential foundation to better understand the underlying causes of Charcot-Marie-Tooth (CMT) disease and other neurodegenerative diseases.
The study titled “Cryo-electron tomography reveals that dynactin recruits a team of dyneins for progressive motility,” appeared in the Nature Structural & Molecular Biology.
The dynein family of proteins drives the transport of cargo inside cells, taking organelles and vesicles and molecules to different parts of the cell along a structure called microtubules. Dyneins convert chemical energy to force and movement, and form a complex with a protein called dynactin. If their function is impaired, cells are not able to divide and people can develop neurological diseases.
The study provided 3D data showing for the first time that dynactin forms a complex with two dyneins in the microtubules.
“If you want a team of horses to move in one direction, you need to line them up,” Gabriel C. Lander, PhD, one of the study’s senior authors and a professor at The Scripps Research Institute, said in a press release. “That’s exactly what dynactin is doing to dynein molecules.”
Scientists knew that dynein only becomes active upon binding to dynactin, but visualizing the complex was difficult due to its flexibility. “We needed to visualize these dynein-dynactin complexes to fundamentally understand how it works to transport molecules,” Danielle Grotjahn, a co-first author on the study, said.
The research team used a technique called cryo-electron tomography to obtain a 3D reconstruction, or tomogram. They developed advanced computational algorithms to achieve greater resolution and clarity. However, the identification of specific components of the dynein-dynactin complex was only made possible after a new strategy to reconstruct the whole microtubule-bound complex.
Investigators expected to find only one dynein molecule bound to dynactin. Instead, they observed that the complex has two dyneins. “These findings provide structural insights into a previously unknown mechanism for dynein regulation,” the researchers wrote.
As each dynein molecule has two motor domains, the discovery means that the complex has a total of four motor domains.
“This discovery was totally unexpected, and will change how this motor complex is represented in cell biology and biochemistry text books,” Saikat Chowdhury, PhD, another of the study’s co-first authors, added.
The images also revealed where dynactin fits in to activate dyneins’ ability to move along microtubules. The study helps explain how dyneins are able to transport large cellular cargos within the cells.
Understanding the assembly and organization of the dynein-dynactin complex is essential to better understand the molecular basis of dynein-related disorders such as CMT disease and spinal muscular atrophy.
The 3D imaging strategy could now be used to visualize other protein complexes, Grotjahn observed. “As we learn more about the 3D organization and architecture of these molecular machines, we will be better equipped to understand how they malfunction in disease,” she added.