Pharnext Conducts Phase 3 Trials to Investigate PXT3003 as Treatment for CMT1A
Written by |
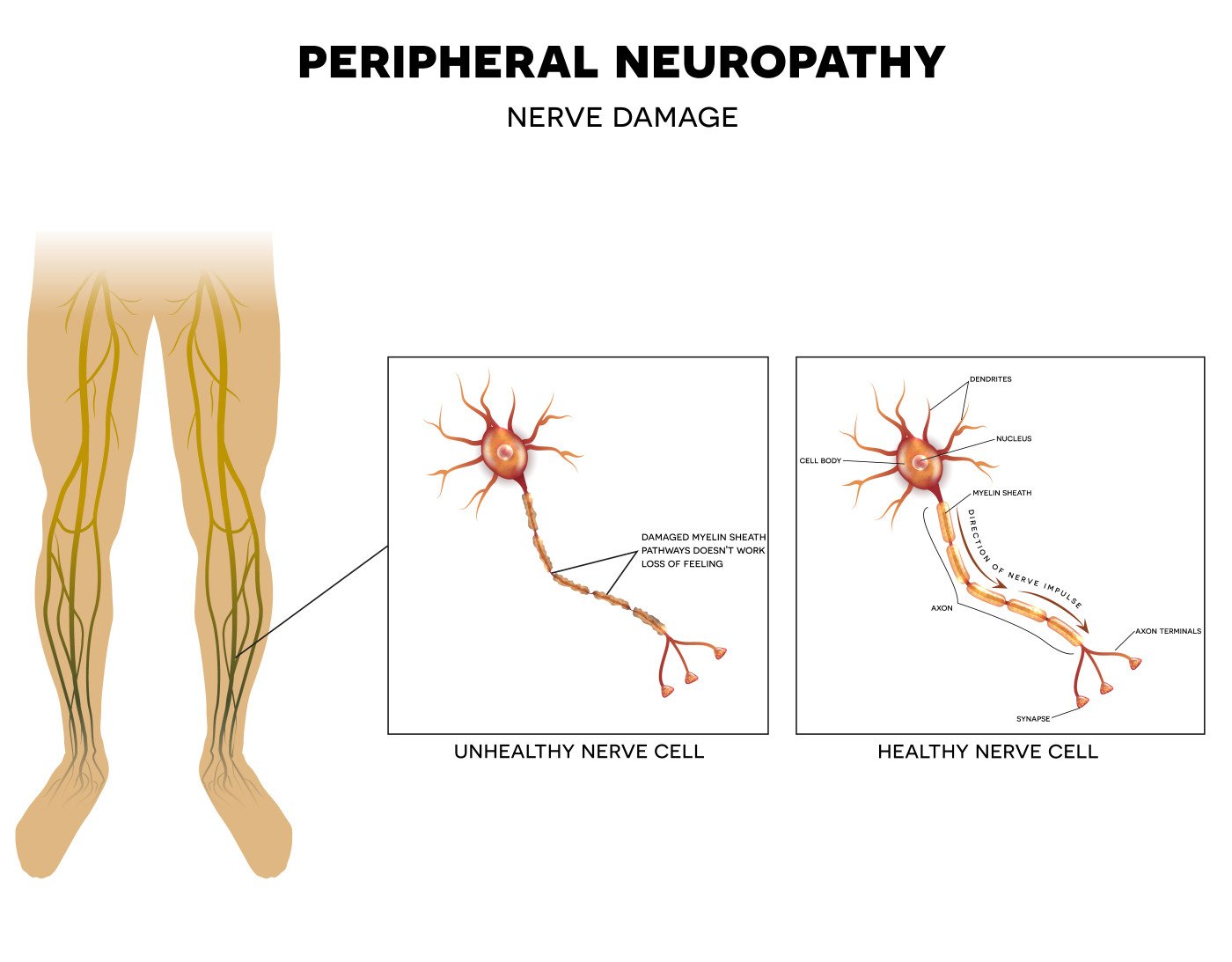
Pharnext presented updates on its Phase 3 PLEO-CMT trial (NCT02579759) evaluating its investigative product PXT3003 as a potential treatment for Charcot-Marie-Tooth type 1A disease (CMT1A) at the 2017 Peripheral Nerve Society Meeting, July 8-12 in Barcelona, Spain.
Data was orally presented by the company’s chief medical officer, René Goedkoop, MD, in a presentation titled “Status of the ongoing multi-center, randomized, double-blind, placebo controlled, pivotal phase III study to assess the efficacy and safety of PXT3003 for CMT1A (PLEO-CMT).”
There were also three poster presentations from the study.
The ongoing PLEO-CMT trial with 323 patients with mild to moderate CMT1A is evaluating the safety and effectiveness of PXT3003 (two doses) compared to placebo.
The study’s primary endpoint is a change in the Overall Neuropathy Limitations Scale (ONLS), a measure of patients’ disability, after 12 and 15 months of treatment. Researchers will also evaluate functional and electrophysiological parameters. Results are expected in the second half of 2018.
The trial will be followed by the PLEO-CMT-FU (NCT03023540) extension study, initiated in March 2017, in which all patients who were treated for the first 15 months will receive PXT3003 treatment for an additional nine months to assess the long-term safety and tolerability of the drug.
PXT-3003 is a novel oral fixed low-dose combination of baclofen, naltrexone and sorbitol, and has been shown to be well-tolerated and effective in improving patients’ disability in a previous Phase 2 study in 80 adults with CMT1A.
In 2014, PXT3003 was granted orphan drug status by the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) for the treatment of CMT1A.
CMT1A is the most common form of CMT and, according to Pharnext, affects at least 125,000 people in Europe and the U.S. The Charcot-Marie-Tooth Association, based in the U.S., says CMT affects about 2.8 million people worldwide.
This condition is caused by a duplication of the PMP22 gene, which encodes myelin, a protein that plays a crucial role in the conduction of neuronal electrical signals. Gene duplication leads to destruction of myelin, which causes loss of nerve conduction. This deficit is the mechanism underlying the progressive muscle atrophy seen in the legs and arms of CMT1A patients, who require wheelchairs in at least 5 percent of cases. CMT almost never affects brain function.





